
Holistic. Transparent. High-quality.
Shortly About Us.
At Lederer & Partner AG, we transform complex visions into clear, successful results. With decades of experience in engineering, construction, commissioning, qualification, and operation, we develop project models that are precisely aligned with your goals. As your trusted partner, we deliver lean, efficient solutions – to minimize risks and maximize value for you.
Our range of services extends from business plans and concepts to planning, audits, and training, all the way to implementation – with comprehensive expertise in pharmaceutical production facility construction, in GMP cleanrooms, or in industrial
automation. Whether it’s about optimizing processes or closing critical gaps – Lederer & Partner brings clarity, structure, and excellence to every phase of your project.
Your success is our project.
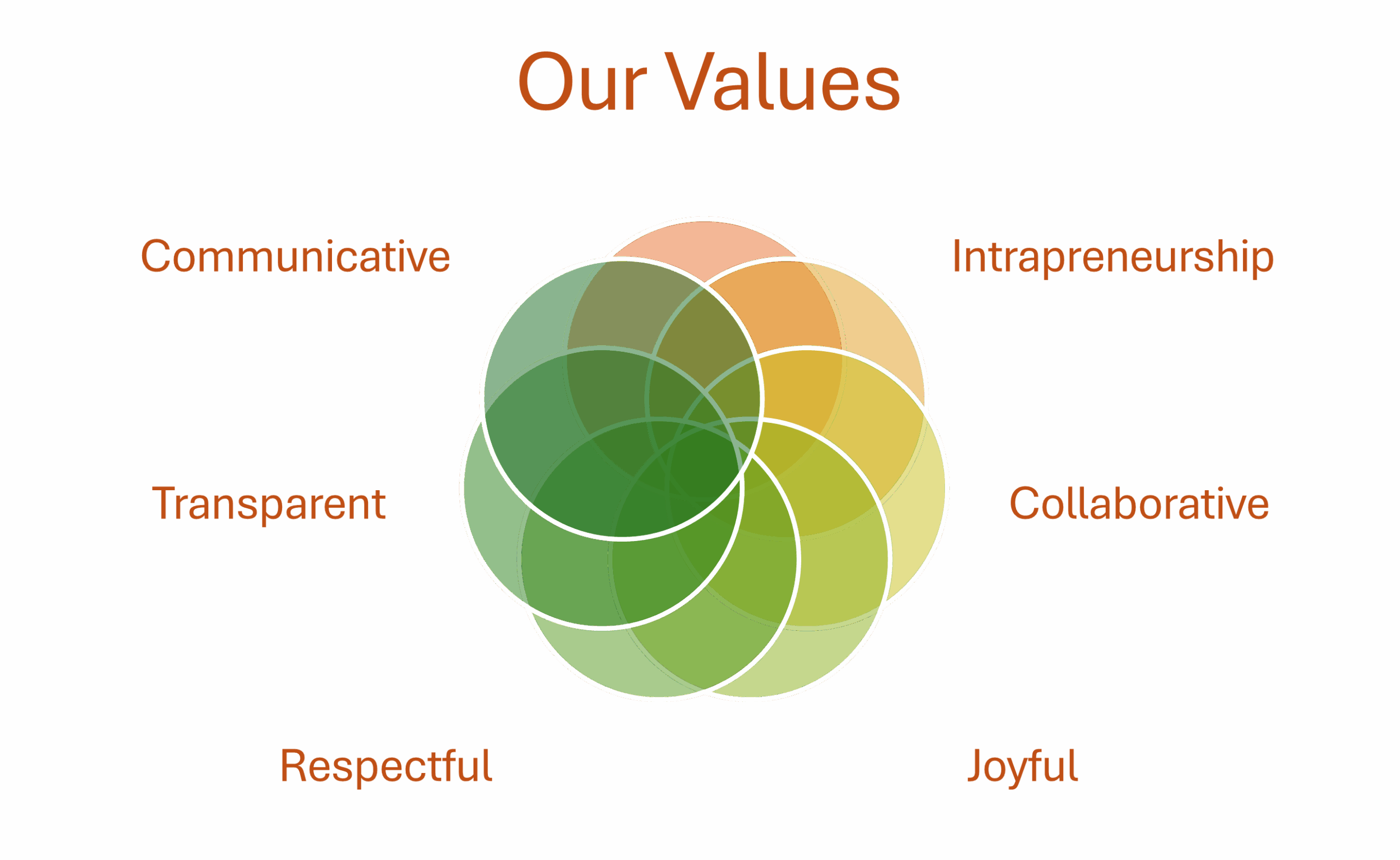
Our Core Belief.
We manage your pharmaceutical investment
projects:
Partnership-based, transparent cooperation between all stakeholders that serves the project objective. Use of innovative, adaptable, successful and customized project management structures. Working in a diverse, inclusive and sustainable team.
Your Partner from Vision to Production:
In pharmaceutical investment projects, we are your trusted specialist—guiding you from the initial idea all the way to operational production. Our expertise, commitment, and collaborative culture create measurable value, inspire confidence, and form the foundation for long-term partnerships grounded in excellence.

People and Joy of Work: Key Drivers of Success
With years of experience, we’ve learned that people—and their joy at work—are the most valuable assets in any project. Committed, motivated teams create extraordinary value for our clients.
Trust, transparency, and open communication foster creativity, ownership, and efficiency. This strengthens team dynamics, boosts resilience, and drives project success— while creating a thriving, positive work environment.
Our Services.
PROJECT &
CONSTRUCTION
MANAGEMENT:
Project Management
Engineering Management
Construction Management
Lean Construction
Integrated Project Delivery (IPD)
EXPERT
CONSULTING &
ANALYSIS:
Strategic Consulting
Operational Excellence (OPEX)
Project Environment Analyses
Technical Audits

QUALITY &
COMPLIANCE
SERVICES:
Commissioning, Qualification & Validation (CQV)
Quality Assurance (QA)
GMP Audits
Regulatory Compliance
We support your project across the entire product life cycle—from CAPEX to OPEX, including technology transfer.
Customized Processing Model.
Built Around Your Goals
From traditional HOAI or SIA models (DBB) to Integrated Project Delivery (IPD),
we design your project structure to align perfectly with your objectives and requirements.
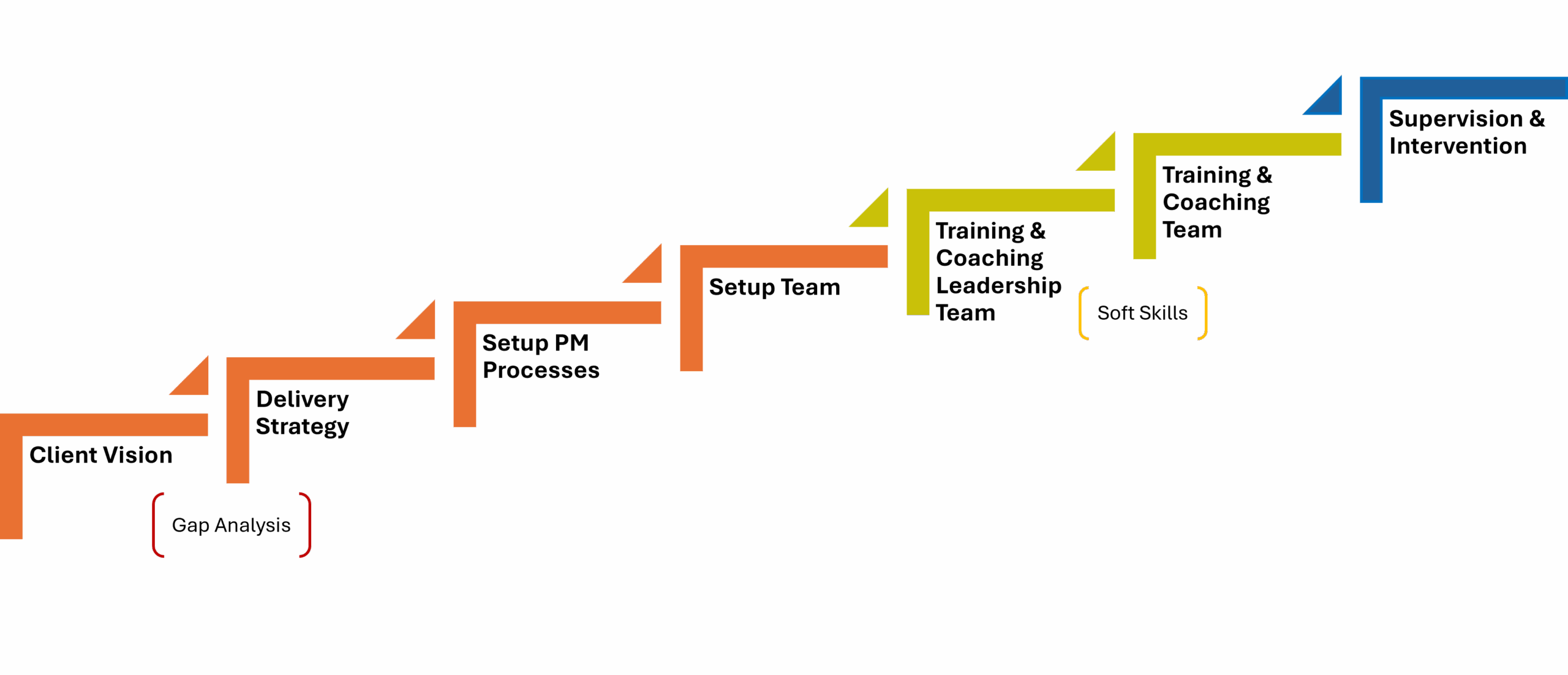
Our Customized Project Delivery Process
Defining a clear vision and measurable goals.
Developing a delivery model tailored to your needs.
Planning all project phases and key milestones.
Coordinating all stakeholders in effective meetings.
Training and Coaching the team for project readiness.
Add Value with ongoing mentoring throughout implementation.
Audits, Assessments & Workshops.

Audits
GMP Audit Process
GMP Audit Process Utilities
GMP Audit Cleanroom & HVAC
ASSESSMENTS
GAP Analysis „ongoing operation“
GAP Analysis planning phase – stage gate reviews
Value Engineering & Risk Workshops
PROJECT DEVELOPMENT &
RISK WORKSHOPS
Project Development
FS – Sprints
Sustainability & Energy Efficiency
TRAINING & MENTORING
PM trainings (Hard Skills, Soft Skills)
Communication trainings (conflicts, team building,…)
Tool trainings (Lean, Agile, …)
Mentoring & Coaching
Technical Expertise & CQV.
Our Experts
We offer specialized expertise across all key areas of pharmaceutical project execution.
Subject Matter Expert (SME) Fill & Finish / Oral Solid Dosage (OSD),
SME Biopharma,
SME Cleanroom & HVAC Systems,
SME Commissioning & Qualification,
SME Project Management (PM),
SME Engineering Management – Interface Coordination,
SME Construction Management (CM),
SME Industrial Automation,
SME Environment, Health, and Safety (EHS)

Commissioning, Qualification &
Validation (CQV)
Defining the qualification model,
Developing the Validation Master Plan (VMP),
Preparing for qualification activities,
Executing Design Qualification (DQ),
Installation Qualification (IQ),
Factory Acceptance Testing (FAT),
Site Acceptance Testing (SAT),
Operational Qualification (OQ),
Providing support for process validation
Meet Our Team.

- Project Management
- Project Set-up and Initiation
- LEAN-Management and methodologies
- Interdisciplinary Engineering & Design Management
- Cleanroom Facilities and HVAC SME

Andrew Jones OPEX Project Manager
- Leading ATMP & Biologics GMP operations
- Manufacturing SME incl. Tech Transfer, ATMPs & Sterility Assurance
- Project Management incl. tech transfer, infrastructure & licensing
- Compliance Improvement & Auditing

Gaelle Berthoud Sr. Process Engineer
- Biotech process expertise (USP and DSP)
- Process Development
- Process Improvement and innovation
- Single Use expertise
- GMP material introduction strategy

Corrado Melis Sr. Process Engineer
- Process Improvement
- Serialization and tamper evident
- Expert in Qualification (Trackwise, Kneat, etc)
- Emerson, Siemens and Rockwell automation
- SAP and Oracle ERP Expert

Manfred Hutter HSE Expert
- EHS Project Management and project support
- Conducting risk analyses as consultant and moderator
- Consultant for Construction Safety
- Implementation of the Major Accident Ordinance
- Other qualification: fire protection specialist, dangerous goods officer

Fraula Daka Project Manager
- Project Management: Business model design & strategic planning
- CMC Lifecycle: End-to-end drug development (SME)
- Process improvements & KPI optimization (PowerBI, Smartsheet)
- Agile & Lean: Change management and implementation
- Integration: Science-to-business expertise

Christian Fimbel Sr. Constructions Manager
- Construction Management
- Pre-Construction
- Lean & Advanced Construction Practices (Target Value Delivery)
- Technology & Innovation Expertise
- Commissioning & Turnover

- Process Expertise: Biotech & Chemical APIs
- Feasibility Studies & Facility Mapping
- Conceptual Design Development
- Commissioning, Qualification & Turnover
News.
Our latest news about the team and Lederer & Partner AG.
Contact Us.
Get in Touch.
Partner


Refine AG
– IPD, Last Planner, Lean Construction, Bim –
Hardturmstrasse 76
8005 Zürich


Organizations